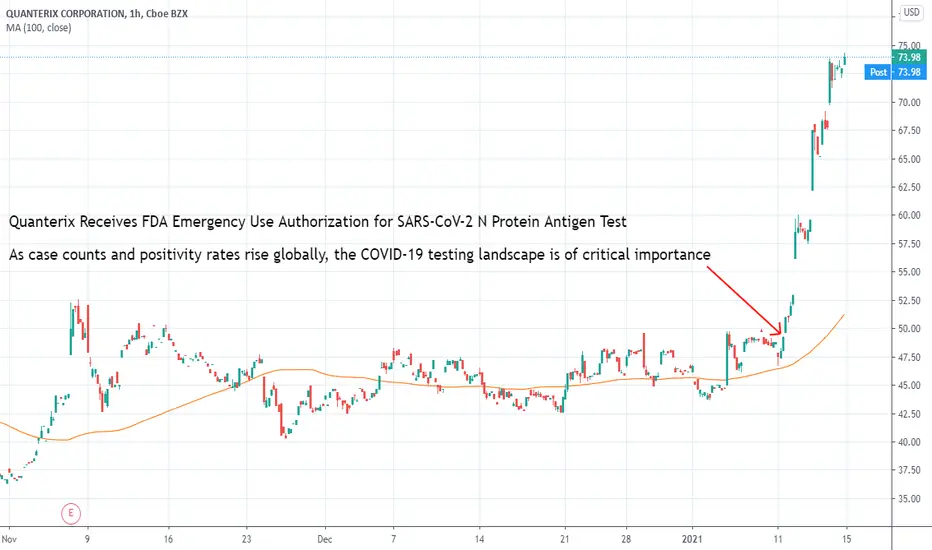
Quanterix Receives FDA Emergency Use Authorization for SARS-CoV-2 N Protein Antigen Test
As case counts and positivity rates rise globally, the COVID-19 testing landscape is of critical importance
finance.yahoo.com/news/quanterix-receives-fda-emergency-authorization-213000735.html
As case counts and positivity rates rise globally, the COVID-19 testing landscape is of critical importance
finance.yahoo.com/news/quanterix-receives-fda-emergency-authorization-213000735.html
Disclaimer
The information and publications are not meant to be, and do not constitute, financial, investment, trading, or other types of advice or recommendations supplied or endorsed by TradingView. Read more in the Terms of Use.
Disclaimer
The information and publications are not meant to be, and do not constitute, financial, investment, trading, or other types of advice or recommendations supplied or endorsed by TradingView. Read more in the Terms of Use.