Long
Major Breakout In $SGEN
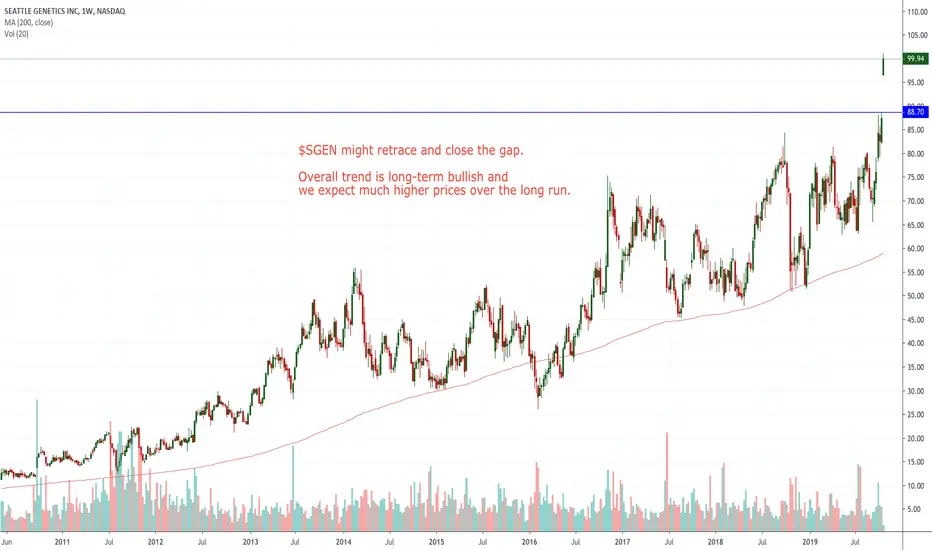
Seattle Genetics (NASDAQ:SGEN) is up in reaction to positive results from a Phase 2 clinical trial, HER2CLIMB, evaluating combination of tucatinib, Roche's Herceptin (trastuzumab) and chemo agent capecitabine compared to trastuzumab and capecitabine alone in patients with locally advanced unresectable/metastatic HER2-positive breast cancer.
The study met the primary endpoint of progression-free survival (PFS) at month 48 with 48% less risk of cancer progression or death (hazard ratio = 0.54).
Key secondary endpoints of overall survival (OS) and PFS in patients with brain metastases were also met.
On the safety front, the most common adverse events were diarrhea, palmar-plantar erythrodysaesthesia syndrome (redness, swelling and pain in the palms and/or soles of the feet), nausea, fatigue and vomiting. Serious/life-threatening adverse events included diarrhea (12.9% vs 8.6% in the comparator arm), increased AST (4.5% vs. 0.5%), increased ALT (5.4% vs. 0.5%) and increased bilirubin (0.7% vs. 2.5%). Increases in AST, ALT and bilirubin indicate liver stress/damage.
Based on the successful results, the company intends to unblind the trial and offer tucatinib, a tyrosine kinase inhibitor, to patients in the comparator arm. It also plans to file a U.S. marketing application in Q1 2020.
As always, trade with caution and use protective stops.
Good luck to all!
Disclaimer
The information and publications are not meant to be, and do not constitute, financial, investment, trading, or other types of advice or recommendations supplied or endorsed by TradingView. Read more in the Terms of Use.
Disclaimer
The information and publications are not meant to be, and do not constitute, financial, investment, trading, or other types of advice or recommendations supplied or endorsed by TradingView. Read more in the Terms of Use.