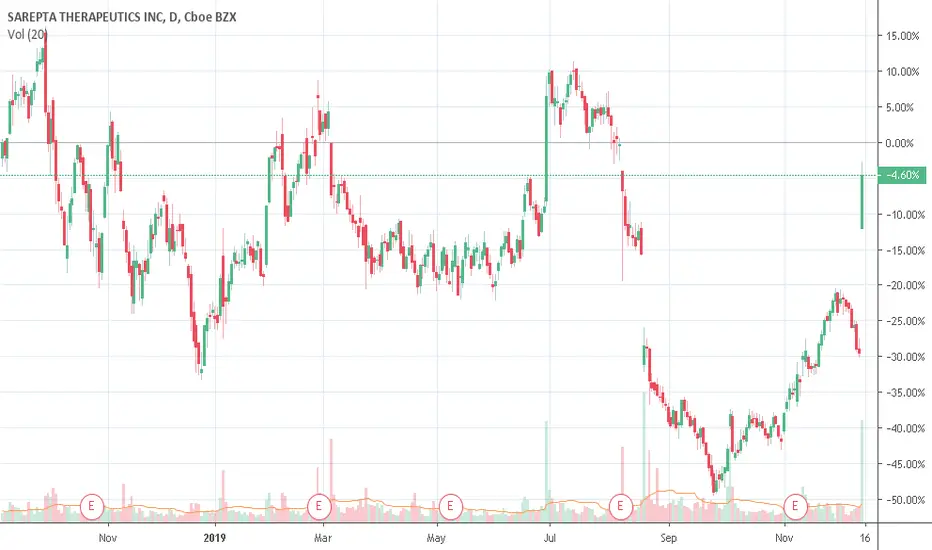
SRPT: Sarepta Therapeutics, Inc.
2019-12-12 18:19:20
Sarepta Therapeutics Announces FDA Approval of VYONDYS 53™ (golodirsen) Injection for the Treatment of Duchenne Muscular Dystrophy (DMD) in Patients Amenable to Skipping Exon 53
2019-12-12 18:19:20
Sarepta Therapeutics Announces FDA Approval of VYONDYS 53™ (golodirsen) Injection for the Treatment of Duchenne Muscular Dystrophy (DMD) in Patients Amenable to Skipping Exon 53
Disclaimer
The information and publications are not meant to be, and do not constitute, financial, investment, trading, or other types of advice or recommendations supplied or endorsed by TradingView. Read more in the Terms of Use.
Disclaimer
The information and publications are not meant to be, and do not constitute, financial, investment, trading, or other types of advice or recommendations supplied or endorsed by TradingView. Read more in the Terms of Use.