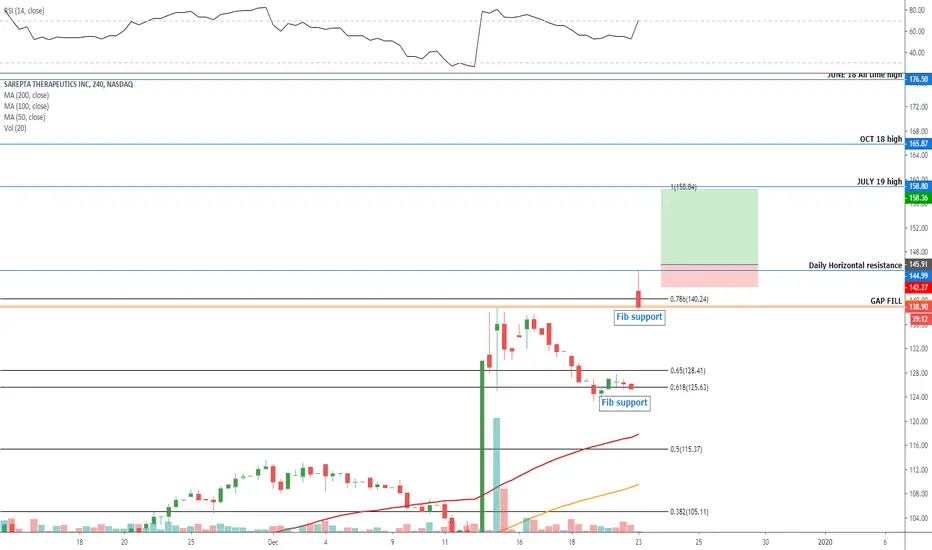
Entry level $146 = Target price $158 = Stop loss $142
We have been covering this long form the $80 region after a massive selloff as a result of FDA issues but today the company received a boost which is detailed below.
Roche enters $1.15 billion licensing deal for Sarepta gene therapy
ZURICH (Reuters) - Roche <ROG.S> entered into a $1.15 billion licensing agreement with Sarepta Therapeutics <SRPT.O> to obtain the right to launch and commercialize Sarepta's investigational gene therapy for Duchenne muscular dystrophy (DMD) outside the United States.
Roche will make an upfront payment of $750 million in cash and $400 million worth in equity at closing for Sarepta's investigational micro-dystrophin gene therapy SRP-9001 that is currently in clinical development, the Swiss drugmaker said in a statement on Monday.
In addition, Sarepta is eligible to receive up to $1.7 billion in regulatory and sales milestones, plus royalties on net sales, Roche said, adding the agreement was expected to close in the first quarter of 2020.
Sarepta will continue to be responsible for the clinical development and manufacturing of SRP-9001 while sharing global clinical development costs equally with Roche.
DMD is a rare degenerative neuromuscular disorder, affecting about one in 3,500-5,000 male births worldwide and causing severe progressive muscle loss and premature death, Roche said.
Earlier this month, Sarepta gained U.S. approval for another DMD drug, Vyondys 53.
Sourse Reuters
We have been covering this long form the $80 region after a massive selloff as a result of FDA issues but today the company received a boost which is detailed below.
Roche enters $1.15 billion licensing deal for Sarepta gene therapy
ZURICH (Reuters) - Roche <ROG.S> entered into a $1.15 billion licensing agreement with Sarepta Therapeutics <SRPT.O> to obtain the right to launch and commercialize Sarepta's investigational gene therapy for Duchenne muscular dystrophy (DMD) outside the United States.
Roche will make an upfront payment of $750 million in cash and $400 million worth in equity at closing for Sarepta's investigational micro-dystrophin gene therapy SRP-9001 that is currently in clinical development, the Swiss drugmaker said in a statement on Monday.
In addition, Sarepta is eligible to receive up to $1.7 billion in regulatory and sales milestones, plus royalties on net sales, Roche said, adding the agreement was expected to close in the first quarter of 2020.
Sarepta will continue to be responsible for the clinical development and manufacturing of SRP-9001 while sharing global clinical development costs equally with Roche.
DMD is a rare degenerative neuromuscular disorder, affecting about one in 3,500-5,000 male births worldwide and causing severe progressive muscle loss and premature death, Roche said.
Earlier this month, Sarepta gained U.S. approval for another DMD drug, Vyondys 53.
Sourse Reuters
Disclaimer
The information and publications are not meant to be, and do not constitute, financial, investment, trading, or other types of advice or recommendations supplied or endorsed by TradingView. Read more in the Terms of Use.
Disclaimer
The information and publications are not meant to be, and do not constitute, financial, investment, trading, or other types of advice or recommendations supplied or endorsed by TradingView. Read more in the Terms of Use.