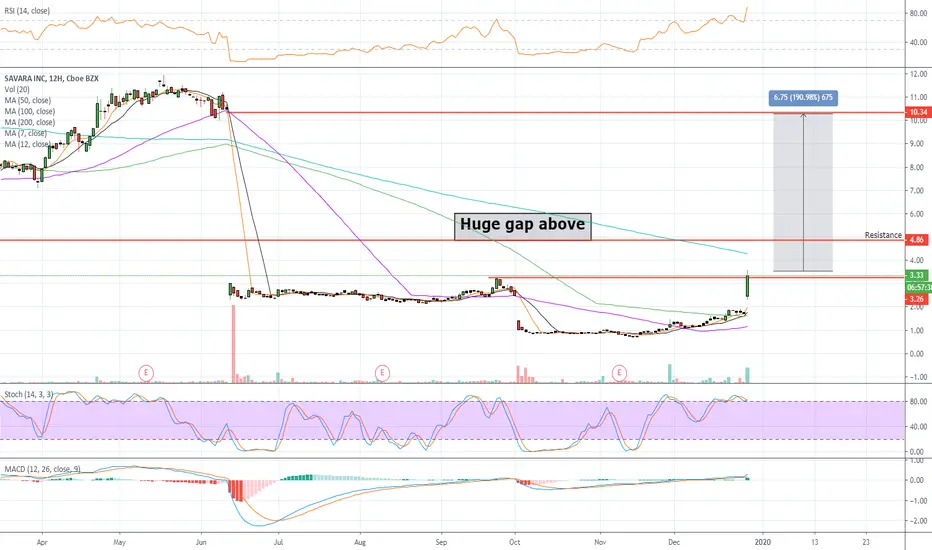
Savara (NASDAQ:SVRA) is up 96% premarket in response to its announcement that the FDA has granted Breakthrough Therapy Designation (BTD) for Molgradex, an inhaled formulation of recombinant human granulocyte-macrophage colony-stimulating factor, for the treatment of autoimmune Pulmonary Alveolar Proteinosis (aPAP).
Breakthrough Therapy status provides for more intensive guidance from the FDA on development, the involvement of more senior agency personnel and a rolling review of the marketing application.
Seeking alpha
Savara, Inc. is a clinical-stage specialty pharmaceutical company, which engages in the development and commercialization of novel therapies for the treatment of serious or life-threatening rare respiratory diseases. Its products include AeroVanc, Molgradex, GM-CSF, and Aironite. The company was founded on April 27, 2017 and is headquartered in Austin, T.
Breakthrough Therapy status provides for more intensive guidance from the FDA on development, the involvement of more senior agency personnel and a rolling review of the marketing application.
Seeking alpha
Savara, Inc. is a clinical-stage specialty pharmaceutical company, which engages in the development and commercialization of novel therapies for the treatment of serious or life-threatening rare respiratory diseases. Its products include AeroVanc, Molgradex, GM-CSF, and Aironite. The company was founded on April 27, 2017 and is headquartered in Austin, T.
⭐⭐⭐ Sign Up for Free ⭐⭐⭐
1) Download our Mobile App >> link-to.app/dexwirenews
2) Join our Telegram >> t.me/DEXWireNews
3) Sign Up for Text Alerts >>
dexwirenews.com/TEXT
4) Follow @DEXWireNews on Social Media
1) Download our Mobile App >> link-to.app/dexwirenews
2) Join our Telegram >> t.me/DEXWireNews
3) Sign Up for Text Alerts >>
dexwirenews.com/TEXT
4) Follow @DEXWireNews on Social Media
Disclaimer
The information and publications are not meant to be, and do not constitute, financial, investment, trading, or other types of advice or recommendations supplied or endorsed by TradingView. Read more in the Terms of Use.
⭐⭐⭐ Sign Up for Free ⭐⭐⭐
1) Download our Mobile App >> link-to.app/dexwirenews
2) Join our Telegram >> t.me/DEXWireNews
3) Sign Up for Text Alerts >>
dexwirenews.com/TEXT
4) Follow @DEXWireNews on Social Media
1) Download our Mobile App >> link-to.app/dexwirenews
2) Join our Telegram >> t.me/DEXWireNews
3) Sign Up for Text Alerts >>
dexwirenews.com/TEXT
4) Follow @DEXWireNews on Social Media
Disclaimer
The information and publications are not meant to be, and do not constitute, financial, investment, trading, or other types of advice or recommendations supplied or endorsed by TradingView. Read more in the Terms of Use.