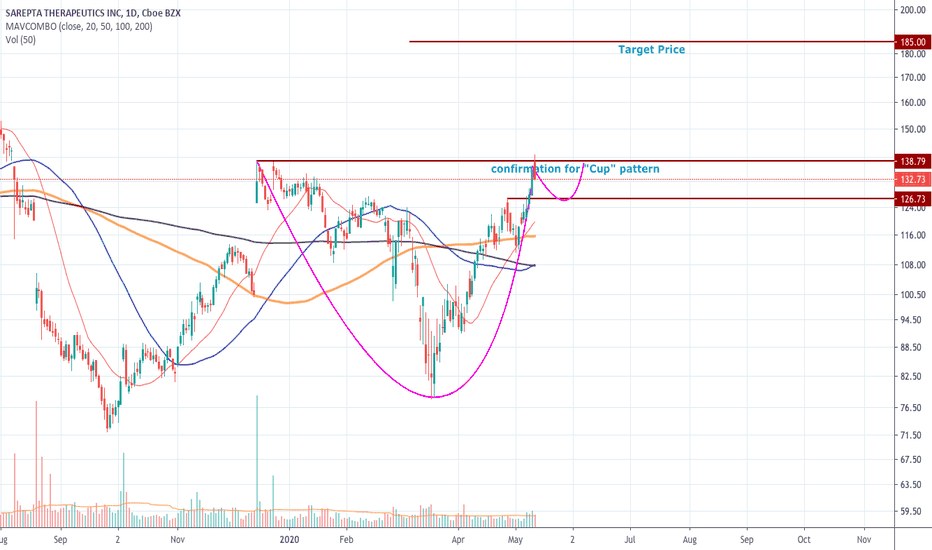
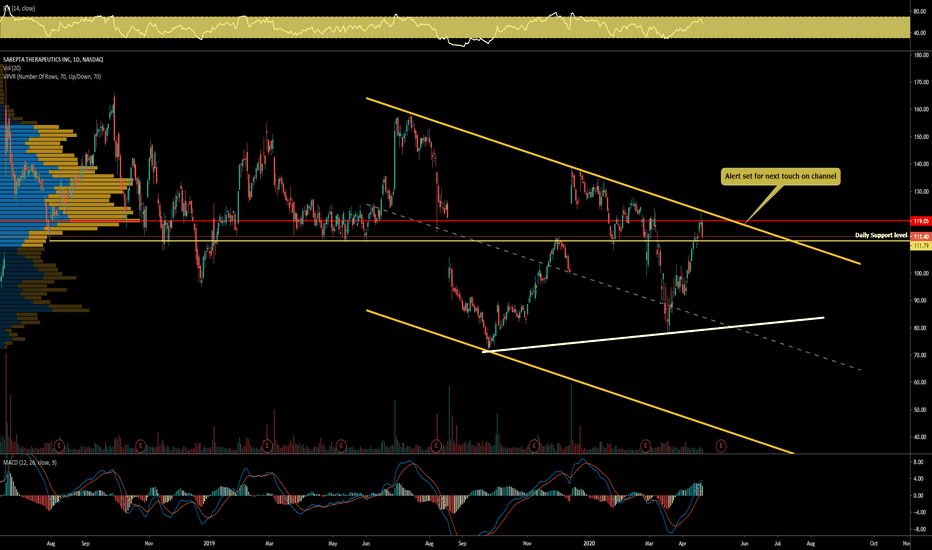
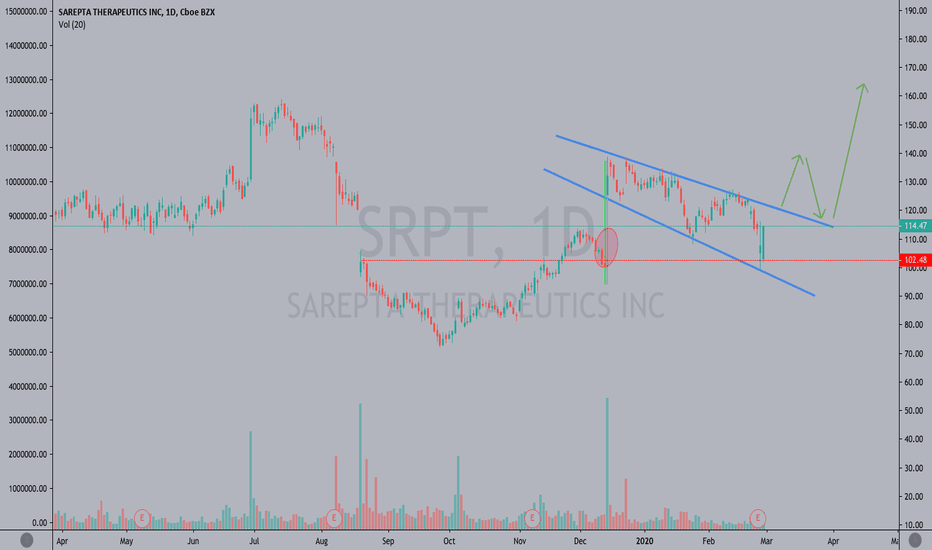
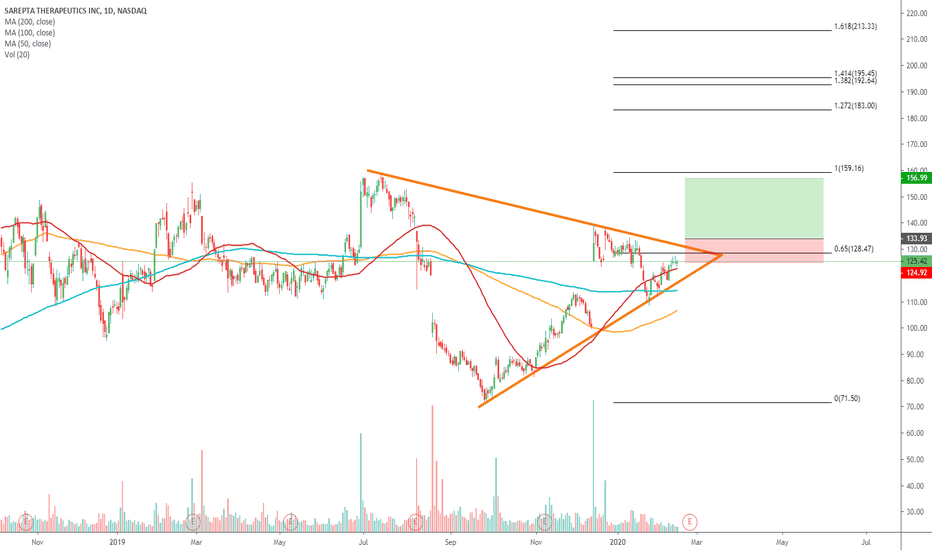
SRPT 16H: XABCD BEARS / 40% correction next(NEW)Why get subbed to to me on Tradingview?
-TOP author on TradingView
-15+ years experience in markets
-Professional chart break downs
-Supply/Demand Zones
-TD9 counts / combo review
-Key S/R levels
-No junk on my charts
-Frequent updates
-Covering FX/crypto/US stocks
-24/7 uptime so constant updates
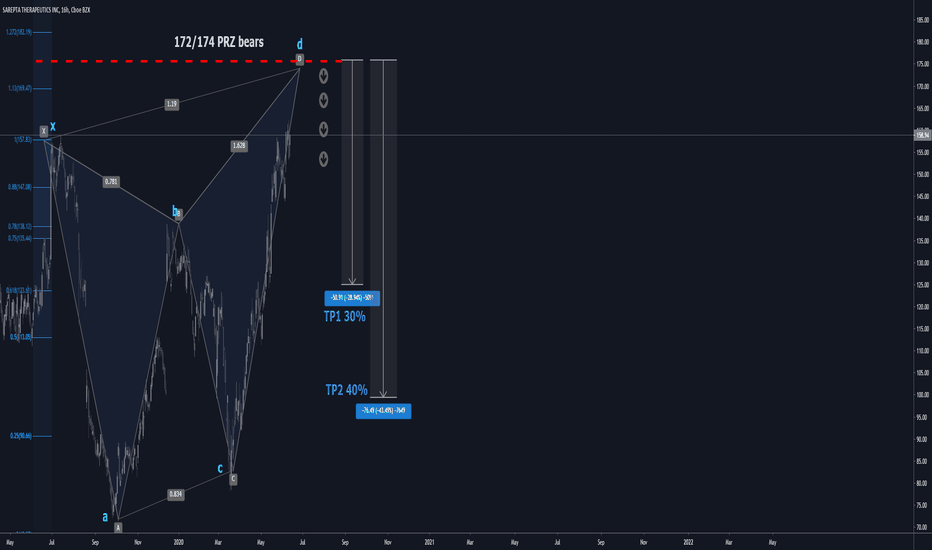
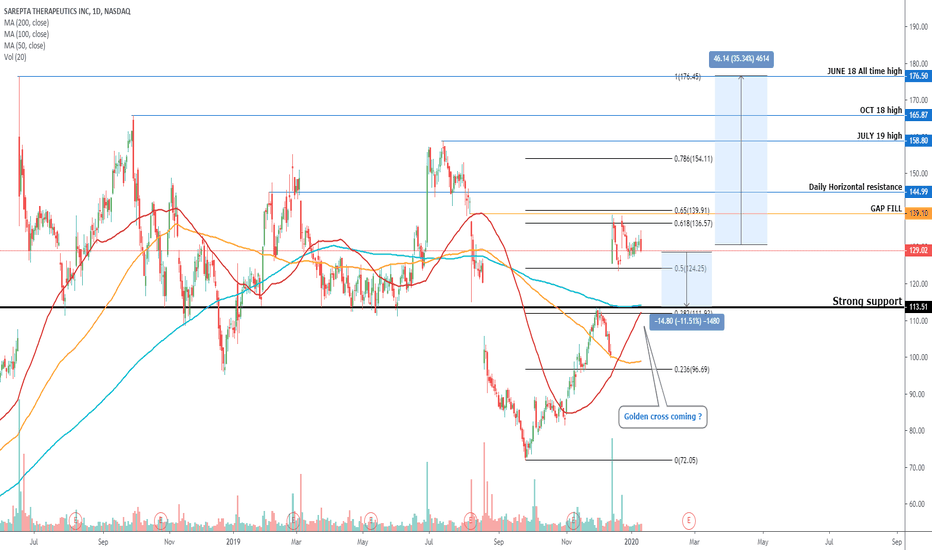
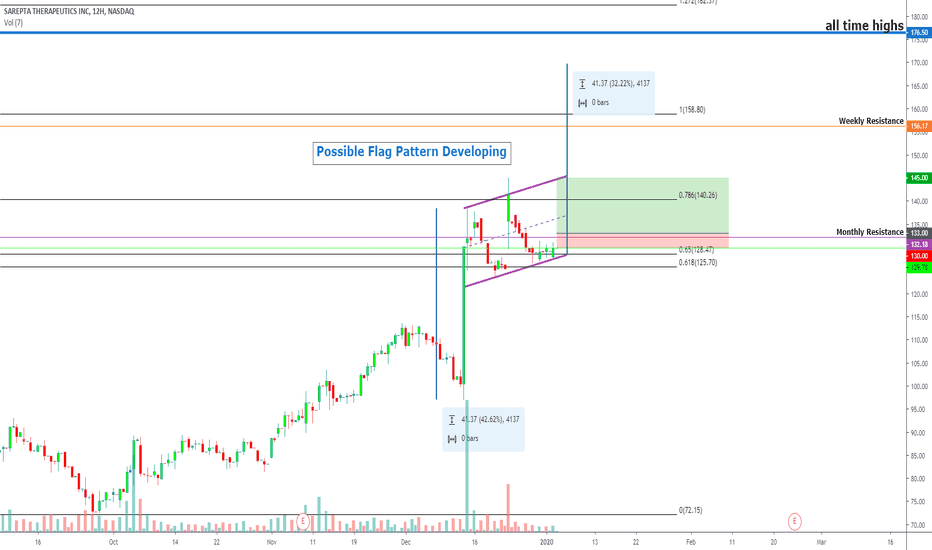
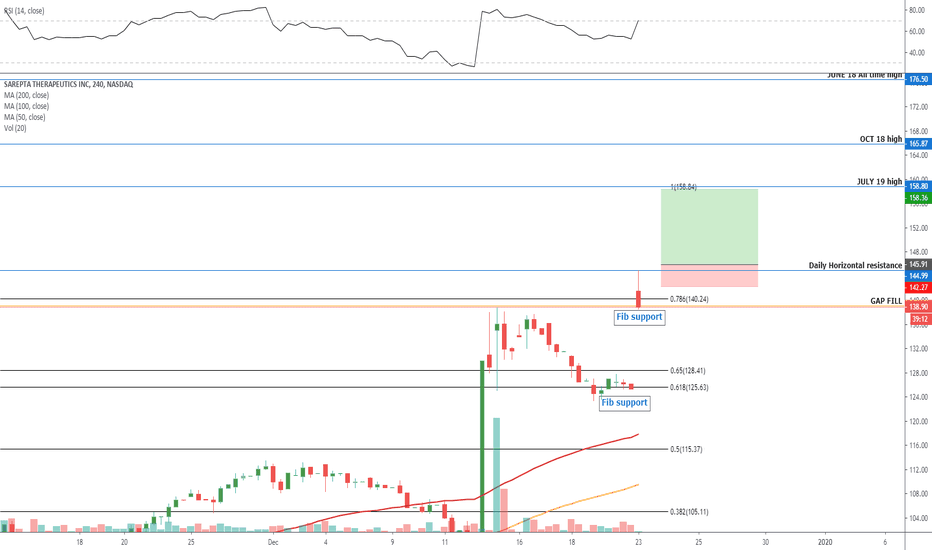
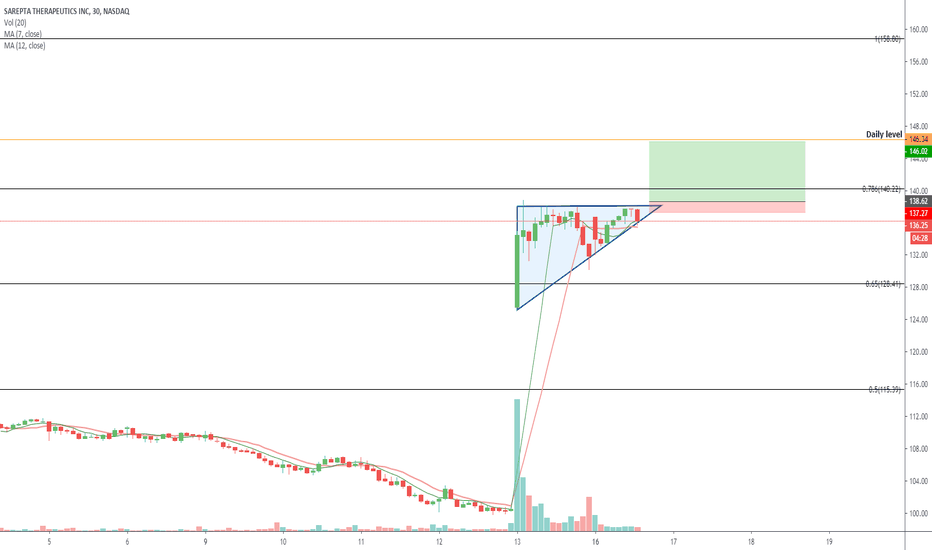
SRPT 16H: XABCD BEARS / 40% correction next(NEW)
IMPORTANT NOTE: speculative setup. do your own
due dill. use STOP LOSS. don't overleverage.
🔸 Summary and potential trade setup
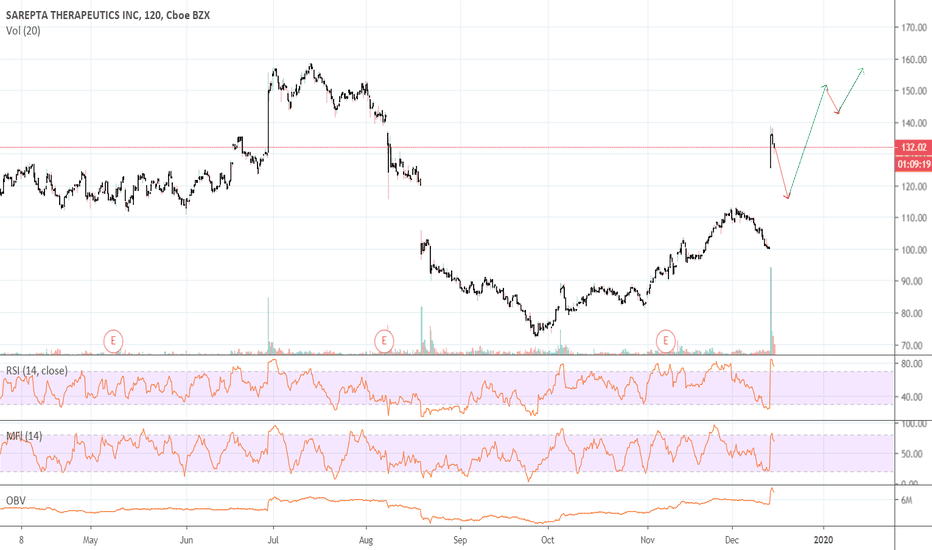
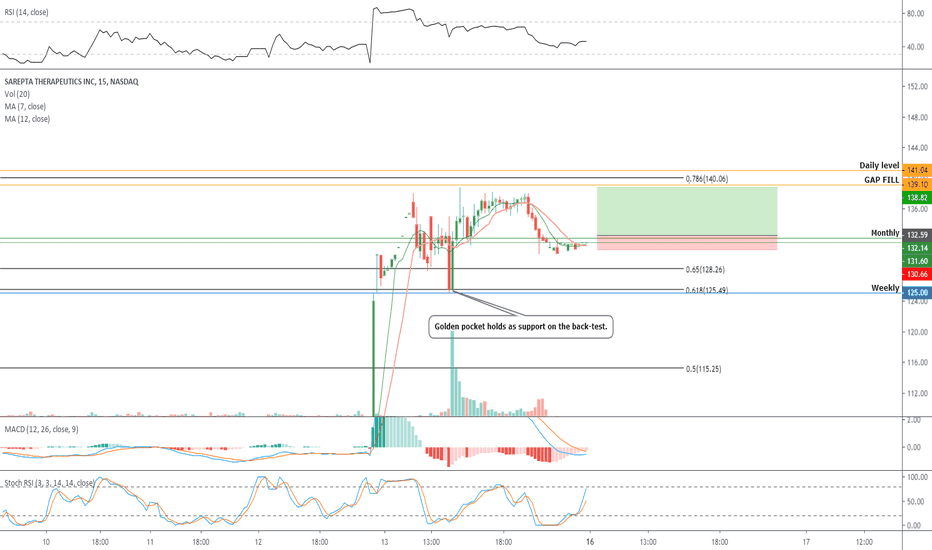
::: SRPT 16hour chart review
::: XABCD BEARS
::: 40% correction up next
::: point D / PRZ near 172/174
::: get ready to short for gains
::: TP1 is 30% gains
::: TP2 is 40% gains
::: strategy: short from PRZ
::: correction can last 4-8 weeks
::: good luck traders
🔸 Supply/Demand Zones
::: N/A
::: N/A
🔸 Other noteworthy technicals/fundies
::: TD9/Combo update: N/A
::: Sentiment: BEARS
::: Sentiment outlook short-term: BEARISH
AB3A trade ideas
$SRPT Sarepta $150 target
Had broken from original descending channel and moving bullishly higher with the entire sector.
Possible short term trade between resistance levels.
Hopefully you caught the original breakout from the channel with a 20% gain.
PLEASE GIVE US A LIKE IF YOU APPRECIATE OUR CONTENT
Sarepta Therapeutics could move big on Monday All eyes and ears will be focused this week on the JPM20 conferences at which SRPT is expected to possibly give a pre-announcement on earnings and a Drug Trial Update on Monday. These events can move a stock very fast and the option market is already implying a 6.5% move in the stock.
Short interest in the company is high at !7% which could led to quiet a rally on any positive updates.
Average Recommendation: BUY Average Target Price: 196.86
23 Buy
2 Overwight
1 Hold
0 Sell
Company Profile
Sarepta Therapeutics, Inc. is a commercial-stage biopharmaceutical company, which is engaged in the discovery and development of therapeutics for the treatment of rare diseases. The company was founded on July 22, 1980 and is headquartered in Cambridge, MA.
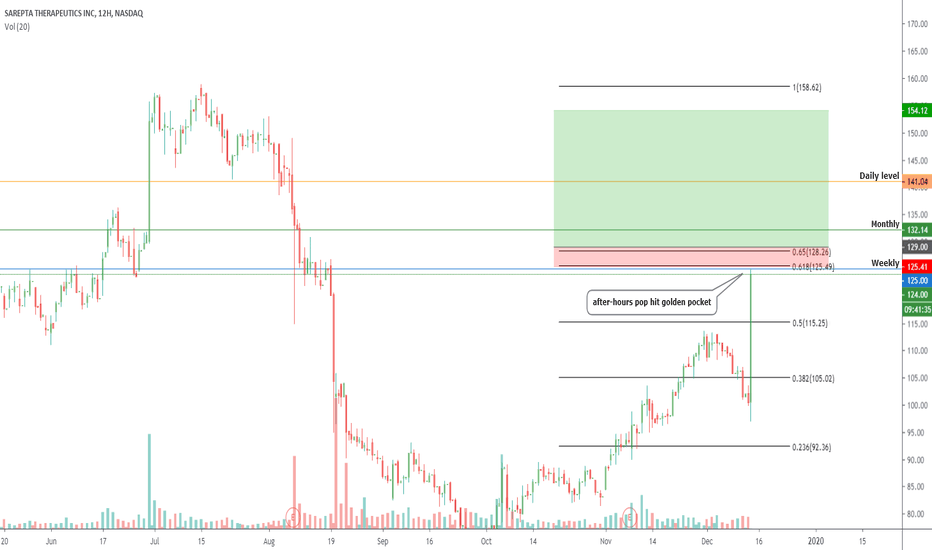
$SRPT Next trade for Sarepta Therapeutics Entry level $146 = Target price $158 = Stop loss $142
We have been covering this long form the $80 region after a massive selloff as a result of FDA issues but today the company received a boost which is detailed below.
Roche enters $1.15 billion licensing deal for Sarepta gene therapy
ZURICH (Reuters) - Roche entered into a $1.15 billion licensing agreement with Sarepta Therapeutics to obtain the right to launch and commercialize Sarepta's investigational gene therapy for Duchenne muscular dystrophy (DMD) outside the United States.
Roche will make an upfront payment of $750 million in cash and $400 million worth in equity at closing for Sarepta's investigational micro-dystrophin gene therapy SRP-9001 that is currently in clinical development, the Swiss drugmaker said in a statement on Monday.
In addition, Sarepta is eligible to receive up to $1.7 billion in regulatory and sales milestones, plus royalties on net sales, Roche said, adding the agreement was expected to close in the first quarter of 2020.
Sarepta will continue to be responsible for the clinical development and manufacturing of SRP-9001 while sharing global clinical development costs equally with Roche.
DMD is a rare degenerative neuromuscular disorder, affecting about one in 3,500-5,000 male births worldwide and causing severe progressive muscle loss and premature death, Roche said.
Earlier this month, Sarepta gained U.S. approval for another DMD drug, Vyondys 53.
Sourse Reuters
$SRPT Sarepta Therapeutics Halted Afterhours. Sarepta Therapeutics (NASDAQ:SRPT) +23.4% after-hours on news the Food and Drug Administration granted accelerated approval to the company's Vyondys 53 (golodirsen) injection to treat a rare Duchenne muscular dystrophy mutation.
In making its decision, the FDA says it considered the potential risks associated with the drug, the life-threatening and debilitating nature of the disease and the lack of available therapy.
As part of the accelerated approval process, the FDA requires SRPT to conduct a clinical trial to confirm the drug's clinical benefit.
Source seeking alpha
Sarepta Therapeutics, Inc. is a commercial-stage biopharmaceutical company, which is engaged in the discovery and development of therapeutics for the treatment of rare diseases. The company was founded on July 22, 1980 and is headquartered in Cambridge, MA.